FDA’s Draft Guidance on Computer Software Assurance for Manufacturing, Operations and Quality System Software has emphasized on ‘Critical Thinking’ to be a crucial part while implementation of automated systems. In brief it is a paradigm shift from document focused computer system validation practices to critical thinking assurance practices. The industry and the FDA are driving the shift toward computer software assurance (CSA).
Traditionally, FDA’s CSV (Computer System Validation) focuses on documentation, followed by testing activities, assurance needs, and critical thinking. Whereas, in the new CSA model it focuses first on critical thinking and then on assurance needs, testing activities, and documentation
CSA Approach:
- Critical Thinking: Risk definition for each Software Features
- Assurance: Demonstrate if the feature & functionality is working as desired
- Testing Activities: Leverage and define the different test methods like Scripted and Unscripted testing
The new FDA guidance intends to focus on more testing and considerably less documentation. Let us have a brief overview of the FDA’s CSA approach for Scripted and Unscripted testing.
Testing Activities – Scripted and Unscripted Testing:
When we go by the definition of Unscripted Testing, it is a type of software testing in which the tester is free to select any possible methodology to test the software. As per the typical definition of unscripted testing, software developers / Testers use their skills and abilities to test the software developed by themselves.
Let’s take an Example of Scripted and Unscripted Testing:
When we consider of LMS (Learning management software) and applying critical thinking
- Training need generation, Training Session management can be considered as unscripted testing.
- The scenario wherein a production executive working in critical area has been failed 3 times in the certification, the executive should be automatically barred from entering the production areas. Since, this has an impact on product quality, we need to check this feature as high risk and must need to execute the proper test script and record the evidence. This scenario will be considered as scripted testing.
During unscripted testing there is always a possibility that few scenarios are missed by the Developers / Testers. So, performing Unscripted testing during the Requirements Phase involving the Business User’s will makes Developers / Testers to test the features, allows them to think more logically and help in adding them as new and required features. To achieve the assurance of such software features, imagine the adoption of LOW CODE PLATFORMS and applying Critical thinking and Unscripted Testing
AmpleLogic Implementation Model: Adoption of Latest Technology TrendsFDA is proposing the adoption of the Smarter Techniques in Software delivery. Research firms like Gartner Predicts that by 2024, low-code application development will be responsible for more than 65% of application development activity
LOW CODE PLATFORMS:
Low-code Delivery approach helps organizations deliver software as efficiently by maximizing reuse and minimizing the need for hand-coding. Freed from the complexities of low-level implementation details, teams can focus on high-level, strategic work that adds business value – with increased creativity and collaboration.
Imagine a situation where business User dictates user stories and the Software service provider develop the software on the fly. The adoption of LOW CODE delivery approach will add value to the CSA Approach.
How Low Code Platform can help you achieve the CSA approach bypassing the Traditional Software service model?
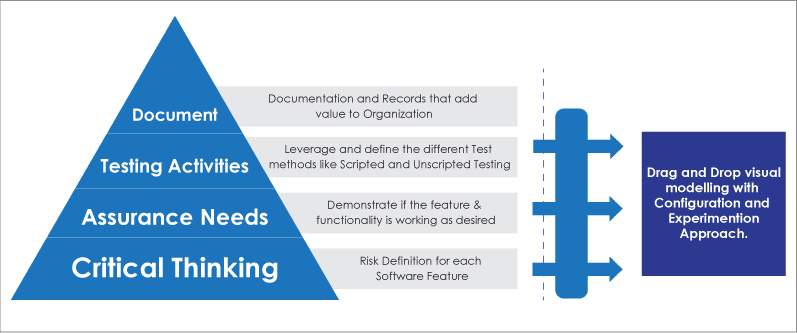
Drag and Drop visual Modelling:
FDA’s CSA approach emphasis on identifying feature, function and operation for the automated system that directly impacts patients or product quality. With Low Code visual modelling the business users can experience the software and are able identify the areas in the process that have direct impact on product quality. Using the Drag and Drop functionality service provider will configure the application and the business user will experience the working functionality. Low Code platform’s ability to breakdown process, workflow, data set, etc. into smaller bits and pieces allows business users to use critical thinking to streamline assurance activities and capture evidence of the processes
Configuration and Experimentation Approach:
The Configuration and Experimentation approach helps in critical thinking and expanding the boundaries of what the software can do. It makes you think more logically and helps in adding more and more required features and functionality. Applying the Configuration and experimentation approach in CSV will also lead to similar outcomes of CSA.
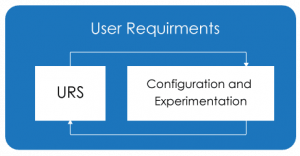
During the assurance phase if the business user’s expectations can be demonstrated by the service provider, then business users can think more logically and add missing system requirements. Following, the high-level system requirement of the business users the service provider can use unscripted testing to identify new features.
“Configuration and Experimentation” of the User Requirements combined with visual modelling (Drag and Drop) Techniques the software service provider along with business users can help us to move toward less documentation, minimizing the validation time and follow the risk-based approach.
Conclusion:
Enhanced Critical thinking + Assurance + Testing Activities (Unscripted Testing) can be achieved thru Configuration and Experimentation while using LOW CODE Development Platforms.
CSA will reduce the Testing and documentation efforts. Following CSA with Low Code Development Platforms and applying Configuration and Experimentation approach will further help in visualizing the software during Requirements Phase itself, reduces the time to deliver.
AmpleLogic has been delivering Services in GMP digitalization with their solutions like Quality Management System (eQMS), Document Management System (DMS), Learning Management System (LMS), QC Planning, Electronic Log Book (eLogs), alongside with other 20+ custom solutions for Biopharma, Pharmaceutical, medical device and related industries. AmpleLogic recognized as Finalist during CPHI Worldwide 2018 Awards for reducing the Validation cycle times with their innovative delivery model.